electron configuration for rn|electron configuration worksheet : Tuguegarao Electronic configuration [Rn] 5f 14 6d 10 7s 2 7p 6: Crystal structure (predicted) FCC . webThis is how to play online slots in eight simple steps. 1) Choose one of our recommended USA online slot sites. 2) Open an account by providing the information and ID documents requested. 3) The slots site will then .
PH0 · rn on the periodic table
PH1 · full electron configuration
PH2 · electron configuration worksheet
PH3 · electron configuration of all elements
PH4 · electron configuration notes ppt
PH5 · electron configuration guide
PH6 · arsenic electron configuration
PH7 · abbreviated electron configuration for cs
PH8 · Iba pa
Resultado da Touro da Bet encontre os melhores jogos de slots e muito mais no seusite. Cadastre-se agora e comece a jogar!
electron configuration for rn*******Mar 23, 2023 Electronic configuration [Rn] 5f 14 6d 10 7s 2 7p 6: Crystal structure (predicted) FCC .electron configuration worksheet A step-by-step description of how to write the electron configuration for Radon (Rn). In order to write the Rn electron configuration we first need to know the number of .
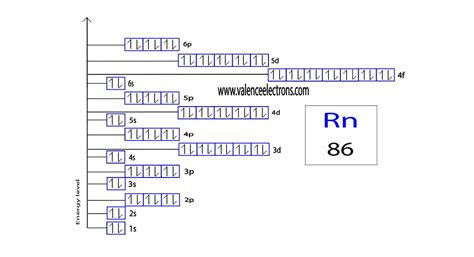
The arrangement of electrons in radon in specific rules in different orbits and orbitals is called the electron configuration of radon. The electron configuration of radon .Electron configuration The arrangements of electrons above the last (closed shell) noble gas. Melting point The temperature at which the solid–liquid phase change occurs. .Bracketed noble gas symbols on the left represent inner configurations that are the same in each period. Written out, these are: He, 2, helium : 1s 2. Ne, 10, neon : 1s 2 2s 2 2p 6. .Radon is a chemical element of the periodic table with chemical symbol Rn and atomic number 86 with an atomic weight of 222 u and is classed as noble gas and is part of . Radon – Electron Configuration and Oxidation States – Rn. Radon is a chemical element with atomic number 86 which means there are 86 protons and 86 .Radon electron configuration. ← Electronic configurations of elements. Rn (Radon) is an element with position number 86 in the periodic table. Located in the VI period. Melting . Orbital Energy Picture of Rn Radon Condensed Electron Configuration. Radon condensed electron configuration is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s . Fluorine Electron Configuration. Neon Electron Configuration. The radon is also the immediate decay product of radium. Its most stable 222 Rn, isotope, has a half-life of only 3.8 days which .Orbital diagram. Radon electron configuration. ← Electronic configurations of elements. Rn (Radon) is an element with position number 86 in the periodic table. Located in the VI period. Melting point: -71 ℃. Density: 0.00923 g/cm 3 . Electronic configuration of the Radon atom in ascending order of orbital energies: 1s 2 2s 2 2p 6 3s 2 3p 6 .The electron configuration of an atom is the representation of the arrangement of electrons distributed among the orbital shells and subshells. . radon (Z = 86), [Xe]6s 2 4f 14 5d 10 6p 6 = [Rn]. In the last row, the 5f orbitals are filled between the 7s and the 6d orbitals, which gives the 14 actinide elements. Because the large number of .
Members of a group typically have similar properties and electron configurations in their outer shell. Period A horizontal row in the periodic table. The atomic number of each element increases by one, reading from left to right. . 211 Rn, 220 Rn, 222 Rn Electron configuration [Xe] 4f 1 4 5d 1 0 6s 2 6p 6 CAS number: 10043-92-2 ChemSpider ID .This page shows the electron configurations of the neutral gaseous atoms in their ground states. For each atom the subshells are given first in concise form, then with all subshells written out, followed by the number of electrons per shell. For phosphorus (element 15) as an example, the concise form is [Ne] 3s 2 3p 3.Tennessine. [Rn]7s 2 5f 14 6d 10 7p 5 [note] Praseodymium. [Xe]6s 2 4f 3. Oganesson. [Rn]7s 2 5f 14 6d 10 7p 6 [note] Notes on the Electron Configuration of particular elements: Dubnium: Value is a guess based on periodic table trend. Seaborgium: Value is a guess based on periodic table trend.Element 86 of Periodic table is Radon with atomic number 86, atomic weight 222. Radon, symbol Rn, has a Face Centered Cubic structure and Colorless color. Radon is a Noble Gas element. It is part of group 18 (helium family or neon family). Know everything about Radon Facts, Physical Properties, Chemical Properties, Electronic configuration .
The electron configuration of francium is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 4f14 5s2 5p6 5d10 6s2 6p6 7s1, if the electron arrangement is through orbitals. . [Rn] 7s 1. When writing an electron configuration, you have to write serially. Try the Electron Configuration Calculator and get instant results for any element. Francium .electron configuration for rn The third major category of elements arises when the distinguishing electron occupies an f subshell. The first example occurs in the case of the lanthanoids (elements having atomic numbers between 57 and 71).The lanthanoids have the general electron configuration [Kr]4d 10 4f i 5s 2 5p 6 5d 0 or 1 6s 2. where i is a number .

Radon is a chemical element which has chemical symbol Rn. The atomic number of radon is 86. It is a colourless, radioactive, tasteless, odourless noble gas. It occurs naturally found in minute quantities as an initial step in the normal radioactive decay chains by which uranium and thorium slowly change into a lead and many other very .The electron configuration and the orbital diagram are: Following hydrogen is the noble gas helium, which has an atomic number of 2. The helium atom contains two protons and two electrons. The first electron has the same four quantum numbers as the hydrogen atom electron ( n = 1, l = 0, ml = 0, ms = + 1 2 ). The arrangement of electrons in nobelium in specific rules in different orbits and orbitals is called the electron configuration of nobelium. The electron configuration of nobelium is [ Rn] 5f 14 7s 2 , . The electron configuration states where electrons are likely to be in an atom. If you don’t have a chart, you can still find the electron configuration. . [Rn] 5f 14 6d 10 7s 2 7p 6. Keep in mind, .Answer: The electron configurations of the elements are presented in Figure 2.2.3, which lists the orbitals in the order in which they are filled. In several cases, the ground state electron configurations are different from those predicted by Figure 2.2.1. Some of these anomalies occur as the 3 d orbitals are filled.The electron configuration of Nobelium is 5f14 7s2. Nobelium is a synthetic chemical element named after Alfred Nobel, the chemist who invented dynamite. It is symbolized by No and its atomic number is 102. Nobelium is a radioactive metal belonging to the transuranic elements as well as mendelevium (remember that the transuranic elements .
Write the noble gas configuration by writing the noble gas core, followed by the valence electrons. A noble gas core is the noble gas element symbol enclosed in brackets: [He], [Ne], [Ar], [Kr], [Xe], or [Rn]. The valence electrons are “leftover” electrons that don’t fill a shell or satisfy the octet rule (except for noble gases) or 18 .Similarly, the observed electron configuration of copper is [Ar]4s 1 3d 10 instead of [Ar]s 2 3d 9. The actual electron configuration may be rationalized in terms of an added stability associated with a half-filled (ns 1, np 3, nd 5, nf 7) or filled (ns 2, np 6, nd 10, nf 14) subshell. Given the small differences between higher energy levels .Electron Configuration -The Electron Configuration of an Element Describes how Electrons are Distributed in their Atomic Orbitals. In Electronic Configuration electrons are arranged in various shells, Subshell and Orbital by following certain rules. To Learn how to Write Electronic Configurations, Detailed Explanation, Filling of orbital with FAQs, .Electron atomic and molecular orbitals A Bohr diagram of lithium. In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. For example, the electron configuration of the neon atom is 1s 2 2s 2 2p 6, meaning that the 1s, 2s, and .
Canais Globo - Conta Globo
electron configuration for rn|electron configuration worksheet